Reveals Molecular Mechanism of Phytochrome phyA Dynamic Acetylation in Regulating Far-Red Light Signal Transduction
Recently, the team led by LIU Xuncheng from the South China Botanical Garden, Chinese Academy of Sciences, published a research paper titled "HDT2-mediated lysine deacetylation promotes phytochrome A degradation during photomorphogenesis in Arabidopsis" inthe renowned plant science journal Molecular Plant. This study elucidates that the stability of Arabidopsis far-red light receptor phytochrome A (phyA) is dynamically regulated by lysine acetylation. The lysine deacetylase HDT2 promotes phyA ubiquitination and subsequent protein degradation by mediating its deacetylation, thereby precisely regulating far-red light signal transduction and seedling photomorphogenesis. This work not only deepens the understanding of the regulatory mechanisms governing photoreceptor stability but also reveals the critical role of non-histone protein acetylation in photomorphogenesis.
1. Research background
Phytochrome A (phyA) is the sole receptor protein in plants specifically perceiving far-red light signals. In dark-grown seedlings, phyA accumulates abundantly but rapidly degrades upon light exposure—a process crucial for seedling light signaling and photomorphogenesis. However, the precise regulatory mechanism controlling phyA stability remains unclear. Lysine (K) acetylation is an evolutionarily conserved post-translational modification. While previous research primarily focused on its role in histone regulation, the function of non-histone acetylation (e.g., on photoreceptors) in photomorphogenesis remains unexplored.
2. Key findings
(1) Acetylomics reveals global deacetylation events during light exposure
Using immunoblot analysis, the team discovered a significant decrease in overall acetylation levels after transferring seedlings from darkness to light. Further systematic acetylomic profiling identified proteins and sites undergoing modification changes. This revealed light-induced deacetylation at numerous proteins and specific lysine residues. Four conserved lysine acetylation sites (K65, K296, K536, and K744) were identified on phyA, with K65 and K744 exhibiting pronounced deacetylation upon light exposure (Figure 1).
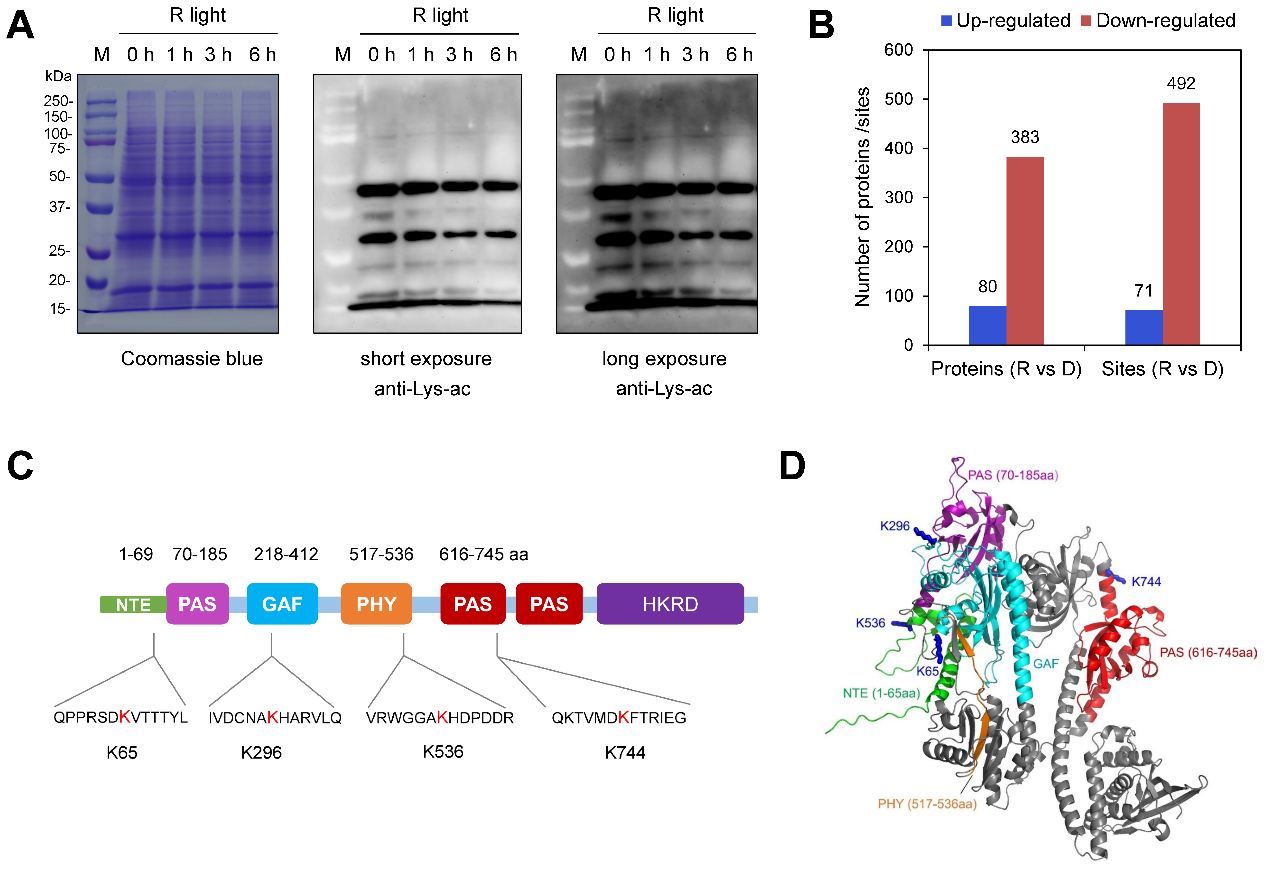
Figure 1. Acetylomic analysis identifies multiple lysine acetylation sites on phyA.(Imaged by LIU et al)
(2) K65 acetylation regulates phyA stability, far-red light signaling, and seedling photomorphogenesis
Using transgenic complementation lines expressing site-specific acetylation-mimic (K65Q) and deacetylation-mimic (K65R) variants, researchers demonstrated that only the acetylation status of K65 affects phyA’s biological function. Further analysis revealed this site also serves as a critical ubiquitination site. Mutation at K65 significantly reduced phyA ubiquitination levels and degradation rates, consequently affecting downstream transcription factor HY5 accumulation and light-responsive gene expression. Co-immunoprecipitation (Co-IP) assays showed that light-induced deacetylation at K65 directly promotes phyA ubiquitination, leading to its degradation via the 26S proteasome pathway. This work first reveals that light-induced phyA degradation requires a "deacetylation-ubiquitination cascade".
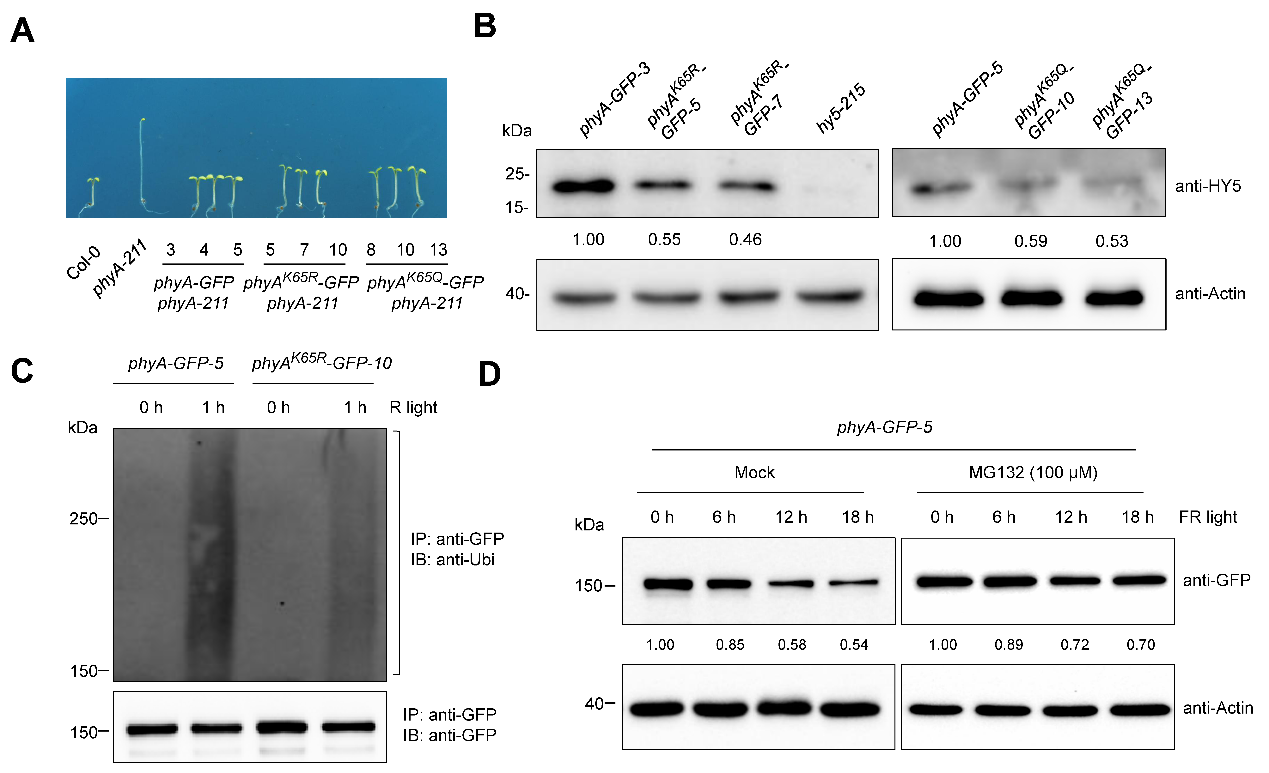
Figure 2. Acetylation modification regulates phyA stability and biological function.(Imaged by LIU et al)
(3) Lysine deacetylase HDT2 mediates phyA deacetylation
Protein-protein interaction assays revealed that the plant-specific lysine deacetylase HDT2 specifically binds to phyA in the nucleus after light exposure. Biochemical analysis demonstrated that HDT2 catalyzes deacetylation at phyA’s K65 site post-illumination, promoting its ubiquitination and degradation. Overexpression of HDT2 accelerated phyA degradation, while knockout of HDT2 suppressed it. Genetic evidence further confirmed that HDT2 specifically participates in phyA-mediated far-red light signaling.
In summary, the team elucidated the core molecular mechanism of the HDT2-phyA regulatory module: In darkness, phyA accumulates in a highly acetylated state. Upon light exposure, nuclear-localized phyA is deacetylated by HDT2, triggering ubiquitination and 26S proteasome-mediated degradation. This cascade precisely controls far-red light signal transduction and photomorphogenesis.
Notably, the team recently published complementary work in Plant Communications (2025) titled "Acetylation-phosphorylation crosstalk regulates phototropin1 kinase activity and phototropism". These collective studies demonstrate that acetylation coordinates with ubiquitination, phosphorylation, and other PTMs to form a multidimensional regulatory network in plant light signaling.
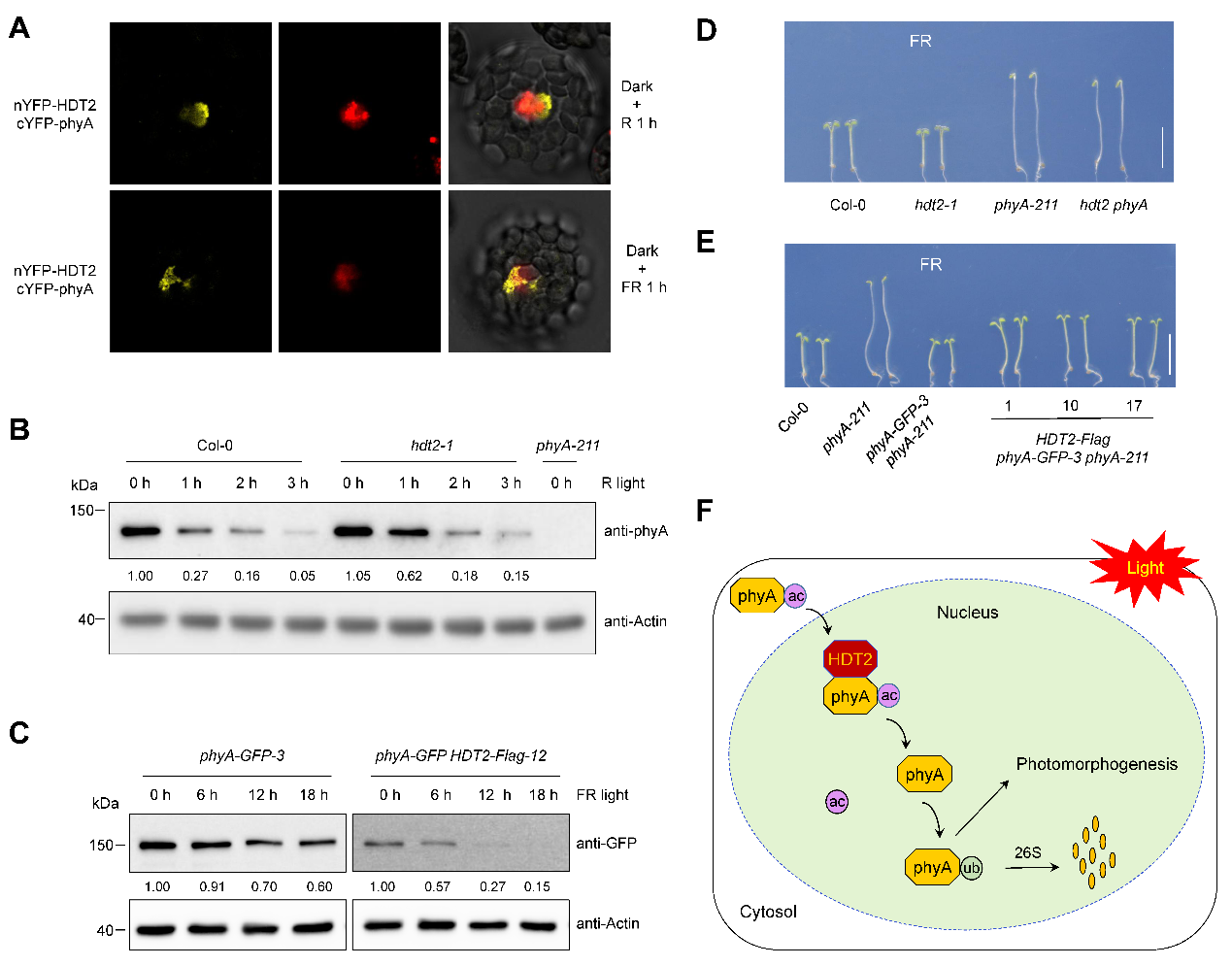
Figure 3. HDT2-mediated phyA deacetylation regulates far-red light signaling and photomorphogenesis.(Imaged by LIU et al)
3. Research Team
ZHENG Feng (Assistant professor), OU Wenli (Master’s student), and DENG Ling (Master’s student) are co-first authors. Professor LIU Xuncheng is the corresponding author. This work is supported by National Natural Science Foundation of China and CAS Youth Innovation Promotion Association Excellent Member Project. Paper Link: https://doi.org/10.1016/j.molp.2025.08.002
File Download: